determination of iron from sample solution by gravimetric method|Chapter 12 Gravimetric Methods of Analysis : sourcing 3. Gravimetric Determination of Iron as Fe2O31. A sample containing iron can be analyzed by precipitation of the hydrous oxide from basic solution, followed by ignition to produce Fe2O3: . Action - STEAMUNLOCKED » Free Steam Games Pre-install.
{plog:ftitle_list}
Para criar uma assinatura online grátis com um fundo transparente, pode utilizar a ferramenta do Photoroom para remover o fundo da imagem da sua assinatura. Vá mais longe com Photoroom. Deseja desbloquear ainda mais funcionalidades para fazer brilhar as suas fotografias? Com o Photoroom Pro, terá acesso a funcionalidades úteis como Editor .
Gravimetric analysis is a quantitative method for accurately determining the amount of a substance by selective precipitation of the substance from an aqueous solution. The precipitate is separated from the remaining aqueous solution by filtration and is then weighed.To determine the amount of iron in a dietary supplement, a random sample of 15 .3. Gravimetric Determination of Iron as Fe2O31. A sample containing iron can be analyzed by precipitation of the hydrous oxide from basic solution, followed by ignition to produce Fe2O3: .As we will discuss during the first week of classes, a sample containing various oxides of iron. can be analyzed by dissolution, oxidation to a single oxidation state, precipitation of the hydrated. hydroxide from basic solution, and finally .
Dissolve a sample after weighing. A precipitating agent with excess amount is added to this solution. The resulting precipitate is filtered, dried (or ignited) and weighed. Determine the .Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate . Iron (II) in the clear liquid is oxidized to iron (III) with excess hydrogen peroxide. Ammonium hydroxide is added to precipitate hydrous iron (III) oxide, which is a gel. The gel is .To determine the amount of iron in a dietary supplement, a random sample of 15 tablets weighing a total of 20.505 g was ground into a fine powder. A 3.116-g sample was dissolved and treated to precipitate the iron as Fe(OH) 3 .
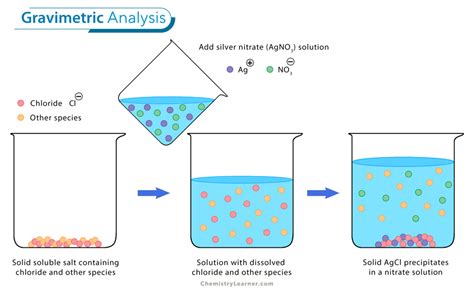
The change in the absorbent’s mass provides a direct determination of the amount of water in the sample. An easier approach is to weigh the sample of food before and after heating, using . xH 2 O by .Gravimetric analyses depend on comparing the masses of two compounds containing the analyte. The principle behind gravimetric analysis is that the mass of an ion in a pure compound can be determined and then used to find the .An example of a gravimetric analysis is the determination of chloride in a compound. In order to do a gravimetric analysis, a cation must be found that forms an insoluble compound with chloride. This compound must also be pure .
Precipitation gravimetry continues to be listed as a standard method for the determination of \(\text{SO}_4^{2-}\) in water and wastewater analysis [Method 4500-SO42– C and Method 4500-SO42– D as published in .A sample lab report is shown at the end of this experiment. Notes: 1. Determine the approximate volume of silver nitrate solution needed by calculating the volume of silver nitrate required IF the unknown was pure sodium chloride. 2. Use a separate stirring rod for each sample and leave it in the beaker throughout the analysis.
After weighing the mixed precipitate, the precipitate is dissolved and the amount of 8-hydroxyquinoline determined by another method. In a typical analysis a 127.3-mg sample of an alloy containing iron, manganese, and other metals was dissolved in acid and treated with appropriate masking agents to prevent an interference from other metals.Ask the Chatbot a Question Ask the Chatbot a Question gravimetric analysis, a method of quantitative chemical analysis in which the constituent sought is converted into a substance (of known composition) that can be separated from the sample and weighed. The steps commonly followed in gravimetric analysis are (1) preparation of a solution containing a known weight .The Gravimetric Determination of Nickel INTRODUCTION Nickel(II) forms a precipitate with the organic compound dimethylglyoxime, C4H6(NOH)2. The formation of the red chelate occurs quantitatively in a solution in which the pH is buffered in the range of 5 to 9. The chelation reaction that occurs is illustrated below. Gravimetric analysis is an analytical technique used for the quantitative determination of an analyte based on the mass of a solid. . quantitative determination of an analyte based on the mass of solid.The element to be identified is precipitated from a solution using this method of analysis by the addition of a suitable precipitating agent .
The bubbling was due to the production of CO 2.. The test of vinegar with potassium carbonate is one type of quantitative analysis —the determination of the amount or concentration of a substance in a sample. In the analysis of vinegar, the concentration of the solute (acetic acid) was determined from the amount of reactant that combined with the solute present in a known .Questions on Sulfate Analysis. Approximately how many mL of 5% BaCl 2 2H 2 O solution would be required to precipitate all the sulfate if we assume that your samples are pure sodium sulfate? Assume that the density of the barium chloride solution is 1.00 g/mL. If the samples were pure potassium sulfate would you require a smaller or larger volume of barium chloride .Gravimetric method with ignition of residue. Gravimetric method with drying of residue. . The absorbance of the barium sulphate solution is measured by a nephelometer or turbidimeter and the sulphate iron concentration, determined by comparison of the reading with a standard curve. . discharging the sample under the surface of solution. If .
Gravimetric Methods of Analysis General aspects, calculations, and typical applications of gravimetric analysis are discussed in Chapter 27, pages 628-641. GRAVIMETRIC DETERMINATION OF CHLORIDE IN A SOLUBLE SAMPLE Introduction The chloride content of a soluble salt can be determined by precipitation of the chloride anion as11. Add 5 mL of 6 M HNO3 to each sample, and boil for a few minutes to ensure that all iron is oxidized to Fe(III). Again, be careful not to boil away all the water! 12. Dilute the sample to 200 mL with distilled water and add 6 M ammonia with constant stirring until the solution is slightly basic (as indicated by a pH probe).unknown solution by using gravimetry. INTRODUCTION Gravimetric analysis is based on the measurement of the mass of a substance of known composition that is chemically related to the analyte. Gravimetric analysis includes precipitation, volatilization .
Analytical Chemistry, Volume 7: Gravimetric Analysis, Part II describes the experimental procedures for the gravimetric analysis of Groups I to V cations. This book is composed of 43 chapters that also present sample preparation, separation, and precipitation protocols. The first six chapters include Group I cations, such as silver, lead, mercury, copper, .Gravimetric methods: The . quantitative methods. that are based on determining the . mass. of a . pure compound . to which the . analyte. is . chemically related. • Precipitation gravimetry: The . analyte. is separated from a solution of the sample as a . precipitate. and is converted to a compound of known composition that can be weighed . Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, 2007) provides an approved method for determining the moisture content of flour. A preweighed sample is heated for one hour in a .This standard prescribes the following methods for determination of various elements in manganese ores: a) Chemical analysis by gravimetric and volumetric method, and b) Atomic absorption method. The gravimetric and volumetric methods are suitable for determination of silica, barium oxide, manganese, iron, phosphorus, sulphur, alumina and other .
custom morso moisture meter
A new, rugged, precise, accurate and fast primary method of measurement has been proposed for the determination of gold in various gold articles. Precise and accurate measurement of gold is the primary requirement for hall marking and to trade gold internationally, as billions of dollars of gold are trading world wide for the various applications. At present Fire . A general principle of gravimetric method of analysis is based on a chemical reaction between analyte and reagent. The analyte (A) of molecules ‘a’ react with the reagent (R) of molecule ‘r’. . This involves either heating the solution or chemically decomposing it in order to separate the target ion from the sample’s solution .If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
5 3. Gravimetric Determination of Iron as Fe2O31 A sample containing iron can be analyzed by precipitation of the hydrous oxide from basic solution, followed by ignition to produce Fe2O3: Fe3+ + (2 + x)H2O FeOOH.xH2O(s) + 3H+ FeOOH.xH2O Fe2O3(s) The gelatinous hydrous oxide can occlude impurities.The pH of the sample solutions should be between 6.5 and 10. (Refer to the additional notes (3) for the explanation). If the solutions are acidic, the gravimetric method or Volhard’s method should be used. Equipment Needed burette and stand 10 and 20 mL pipettes 100 mL volumetric flask 250 mL conical flasks 10 mL and 100 mL measuring cylinders
The titration of the zinc takes less time, and, with ordinary working, is more trustworthy than the gravimetric method. The standard ferrocyanide solution is made by dissolving 43.2 grams of potassium ferrocyanide (K4FeCy6.3H2O) in water, and diluting to a litre. One hundred c.c. are equal to 1 gram of zinc.This method is well suited to the determination of iron in dietary supplements. It is necessary to release the iron ions . Assay of Unknown Sample Solution: Perform the iron assay described above in triplicate using three separate 5.0-mL aliquots of the diluted unknown stock solution. Note that it may require a greater or lesser amount of .This solution will be the vitamin solution you will use for the analysis. Known Iron Sample Preparation The known iron sample requires the dissolving of the solid sample. Heating and filtering are not required. 1) Measure 0.080 g of the known iron sample onto a new piece of tared weighing paper. Transfer this mass into a 50 mL volumetric flask.SPECTROPHOTOMETRY METHOD Bayu Wiyantokoa, Santosob bProfessional Program of Chemical Analysis, Islamic University of Indonesia *Corresponding author Email: *[email protected] April 2, 2018 Abstract The analysis of gold content in concentrate sample us-ing re assay by gravimetric and sample basis cupel loss
Gravimetric analysis is a quantitative method in chemistry that involves determining the amount, or concentration, of a substance present in a sample based on the measurement of its mass. This .
Gravimetric Analysis: Definition, Principle, and Example
Gravimetric Analysis (Heiss)
Gravimetric Analysis
 .jpg)
Resultado da 2 7,1K. 8. 1. Amanda Smell 🤩 pictures and videos on EroMe. The album about Amanda Smell 🤩 is to be seen for free on EroMe shared by gatasbrasileirinhas. Come see and share your amateur porn.
determination of iron from sample solution by gravimetric method|Chapter 12 Gravimetric Methods of Analysis